Unmatched Options of Sizes, Lordotic Angles, & Anchors
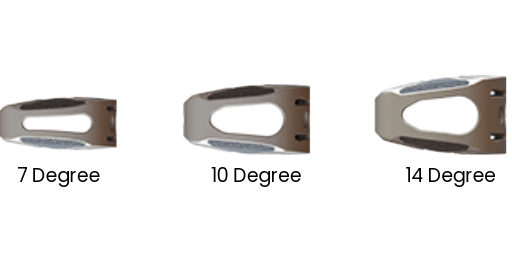
3 LORDOTIC ANGLES
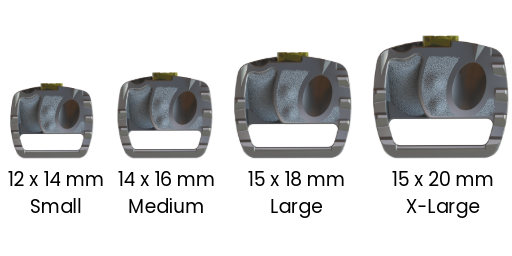
4 FOOTPRINTS
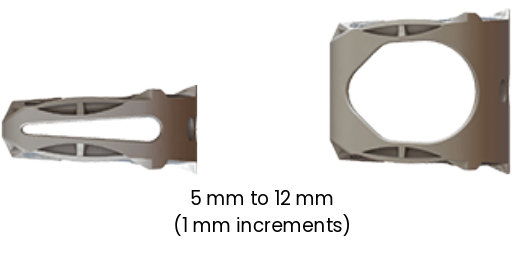
8 HEIGHTS

5 Spacer / Screw Options
System Features
Essential features for the next generation of Cervical Interbody Spacers
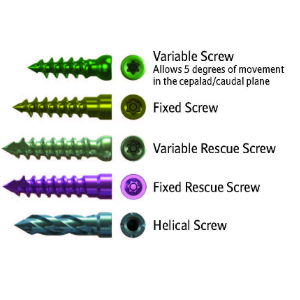
Multiple fixation options including variable, fixed and helical screws.
Screw Options
+ Material: Titanium alloy (Ti6Al4V) per ASTM F136
+ Variable and Fixed Angle Screws – 3.5 mm diameter in lengths of 14mm, 16 mm, 18mm, and 20 mm (T8 Hexalobe Drive Socket)
+ Variable and Fixed Angle Rescue Screws – 3.7 mm diameter in lengths of 14 mm, 16 mm and 18 mm. (T8 Hexalobe drive socket)
+ Helical Screws – 3.5 mm diameter in lengths of 14 mm, 16 mm, 18 mm and 20 mm (Left-hand Thread Drive Socket)

Secure one-step cam locking
The One-Step Cam Locking System provides a simple method to secure bone screws in place.
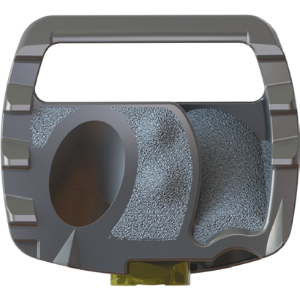
Porous superior and inferior surfaces allow for bone on-growth and in-growth
The porous surfaces around the screw holes allow for enhanced Bone-on-Growth and Ingrowth, essentially acting as a lock washer to further stabilize the spacer.

Edge stabilizers anchor into cortical bone to resist rotation and flexion
The Edge Stabilizers anchor into the cortical bone around the periphery of the cervical endplate to help resist rotational forces.

Lateral windows allow for radiographic assessment of fusion
The lateral aspects of the spacer are open to facilitate radiographic fusion assessment.
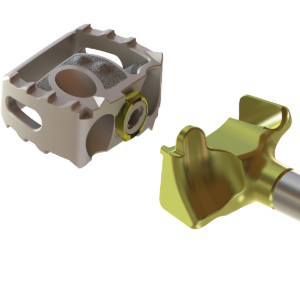
Threaded for Attachments
The anterior face incorporates three holes, the center one threaded, for inserter attachment.

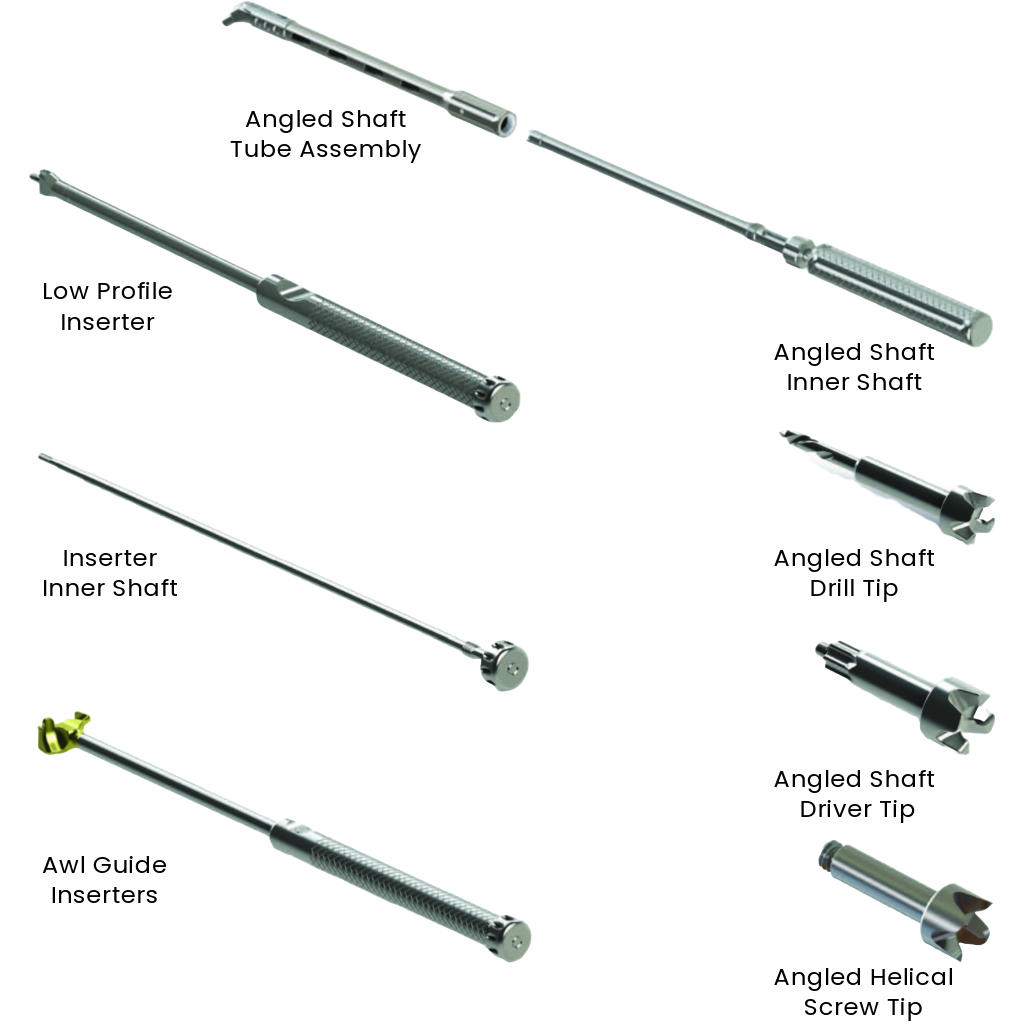
Narrow low profile inserter to enhance visibility during MIS procedures
The narrow tip of the low profile inserter will enhance visibility during MIS procedures.

SureMAX™ Spacers are provided sterile only
The SureMAX™ Family of spacers are available sterile packed to reduce SPD expenses and provide quick easy access for unplanned surgeries.
The SureMAX™ Family of Cervical Spacers
The new standard in interbody design.
Comprehensive set of instrument options to support surgical technique preferences provided in two modular trays.
SureMAX-SA® instrumentation Platform
SureMAX®-SA Instrument Sterilization Tray
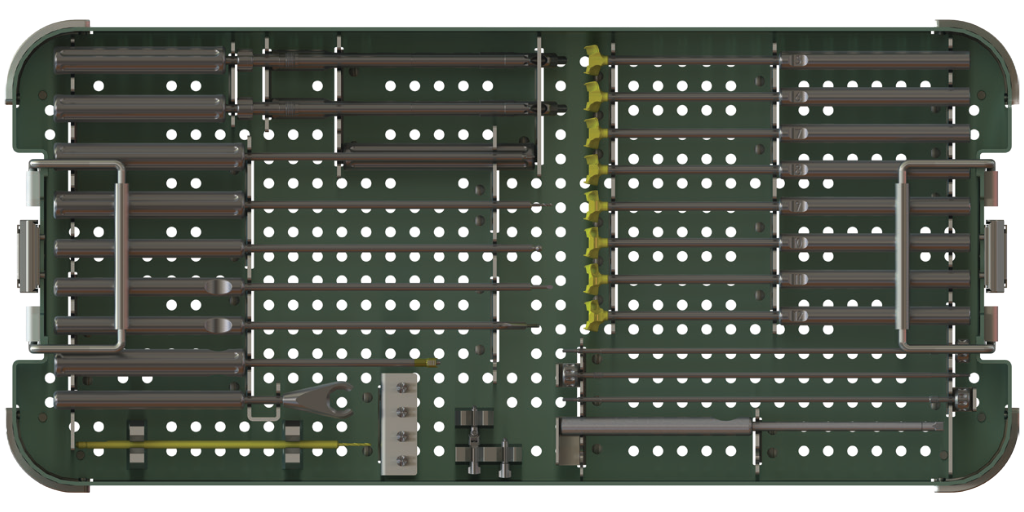
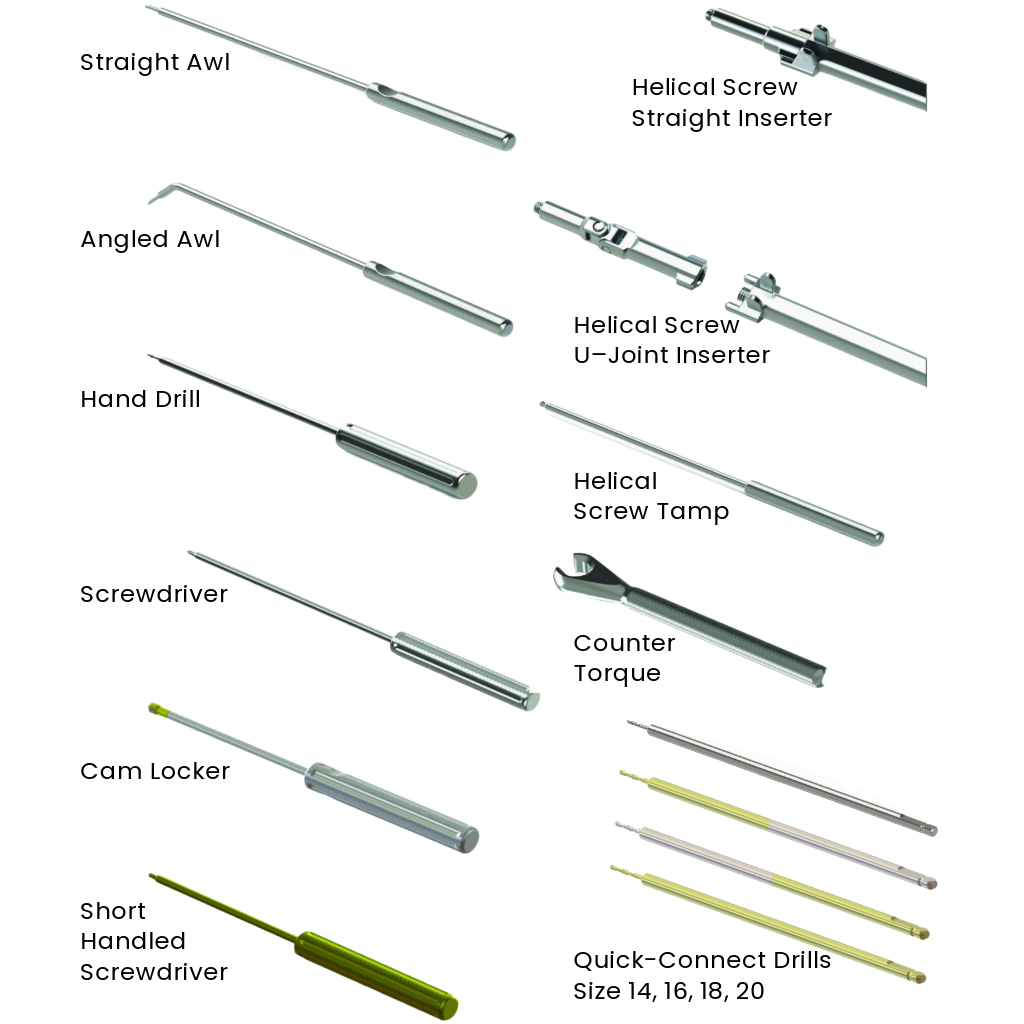
One set of instruments for both SureMAX™ and SureMAX-X™ Cervical Spacers.
SureMAX® cervical spacer instrumentation

Single Modular Tray

Distraction Wedges to help open collapsed disc spaces.

Aggressive Rasps with convex surfaces to strip and shape concavity of vertebral endplates.

Trials match the geometry of the implants for an accurate assessment of size.

Variety of Inserter Tools, with and without guards, allow for single handed tool/implant application.

Patented Slap Hammer design allows rapid insertion/extraction and enhanced visualization of the operative site when using a microscope.
Resources
Brochures & Surgical Technique Guides
Important Information
Description, Indications, and Contraindications
Indications For Use
The SureMAX®-SA Cervical Standalone System is a stand-alone anterior cervical interbody fusion device indicated for use in skeletally mature patients as an adjunct to fusion for the treatment of degenerative disc disease (DDD). DDD is defined as discogenic neck pain with
degeneration of the disc confirmed by history and radiographic studies. These patients should have had six weeks of non-operative treatment prior to treatment with the device. The SureMAX®-SA Cervical Standalone System is to be used with autograft bone graft and/or
allogeneic bone graft composed of cancellous and/or corticocancellous bone and implanted via an open, anterior approach. When used with the fixed or variable screws, the SureMAX®-SA Cervical Standalone System is intended to be used at one or two levels from C2-T1 and requires no additional fixation. When used with one or more helical screws, the SureMAX®-SA Cervical Standalone System is intended to be used at one level and with supplemental fixation.
Contraindications
+ Active local infection in or near the operative region.
+ Active systemic infection and/or disease.
+ Severe osteoporosis or insufficient bone density, which in the medical opinion
of the physician precludes surgery or contraindicates instrumentation.
+ Current metastatic tumors of the vertebrae adjacent to the implant.
+ Known or suspected metal sensitivity.
+ Endocrine or metabolic disorders known to affect osteogenesis (e.g., Paget’s disease, renal osteodystrophy, hypothyroidism).
+ Significant mental disorder or condition that could compromise the patient’s ability to remember and comply with preoperative and postoperative instructions.
+ Neuromuscular disorder that would engender unacceptable risk of instability, implant fixation failure, or complications in postoperative care. Neuromuscular disorders include spina bifida, cerebral palsy, and multiple sclerosis.
+ Pregnancy.
+ Patients unwilling or unable to follow postoperative instructions,
especially those in athletic and occupational activities.
+ Morbid obesity.
+ Symptomatic cardiac disease.
+ Skeletal immaturity.
+ Grossly distorted anatomy.
+ Any condition not described in the Indications for Use.
Warnings and Precautions
Warnings
+ Surgery is not always successful. Preoperative symptoms may not be relieved or may worsen. Surgical knowledge of the procedure and the device are important, as is patient selection. Patient compliance is also important. Tobacco and or alcohol abuse may lead to unsuccessful results.
+ Implants are single use only and should not be reused. Once a device has been implanted, it must never be reused. Reuse of a single use device that has come in contact with blood, bone, tissue or other body fluids may lead to patient or user injury. Possible risks associated with reuse of a single use device include, but are not limited to, mechanical failure and transmission of infectious agents.
+ Appropriate endplate preparation and device selection is needed to obtain proper fit.
+ Delayed healing can lead to fracture of the implants due to increased stress and material fatigue. Patients must be fully informed of all the risks associated with the implant and the importance of following postoperative instructions regarding weight bearing and activity levels to facilitate proper bone healing.
+ The implant must be handled carefully, following the manufacturer’s instructions, to prevent damage to the implant.
+ Implants must not be modified or otherwise processed in any way.
+ Care must be taken to avoid using dissimilar metals, in contact with one another, as corrosion may occur. Additional fixation instrumentation that is used to stabilize the affected level must be made of compatible materials, such as Titanium or Titanium alloy. Corrosion may accelerate metal fatigue and lead to failure of the implant.
+ This surgical procedure requires the use of the screw fixation provided to stabilize the fusion site.
Precautions
+ The implantation of an intervertebral body fusion device should be performed only by experienced spinal surgeons with specific training in the use of this device because this is a technically demanding procedure presenting a risk of serious injury to the patient.
+ Based on the fatigue testing results, the physician/surgeon should consider the level of implantation, patient weight, patient activity level, other patient conditions, etc. which may impact on the performance of the system.
+ The surgeon must have a thorough knowledge of the mechanical and material limitations of surgical implants made of Titanium Alloy and be thoroughly familiar with the surgical technique for implanting the SureMAX®-SA Cervical Standalone System for the given Indications for Use.
+ The surgeon should be familiar with the various devices and instruments and verify that all are available before beginning the surgery. Additionally, the packaging and implant should be inspected for damage prior to implantation.
+ In the event that removal of the implant is considered (e.g. due to loosening, fracture, migration of the implant; infection; increased pain, etc.), the risks versus benefits must be carefully weighed. Such events can occur even after healing, especially in more active patients. Appropriate postoperative care must be given following implant removal to avoid further complication.
+ Implants must be fully seated within the inserter prior to use. Care must be taken not to over-tighten the implant-inserter assembly. Additionally, care must be taken not to manipulate the inserter implant interface in a way not recommended by the surgical technique.
+ The surgeon must ensure that the implant is properly seated prior to closing of the soft tissue.
+ Extreme caution must be used around the spinal cord, nerve roots and blood vessels.
MRI Safety Information
The SureMAX®-SA Standalone System has not been evaluated for safety and compatibility in the MR environment. It has not been tested for heating, migration, or image artifact in the MR environment. The safety of these implants in the MR environment is unknown. Scanning a patient who has this device may result in patient injury.
Potential Adverse Effects
Potential complications and adverse effects for this system are similar to those of other spinal instrumentation systems and include, but are not limited to: pseudarthrosis, insufficient bone stock, painful bursa, pressure necrosis, palpable components, early or late loosening of the components; disassembly, bending or breakage of any or all of the components; foreign body (allergic) reaction to the implants; infections possible requiring removal of devices; loss of neurological function, including paralysis, spinal cord impingement or damage.


