Unmatched Options In Cervical Spacer Design
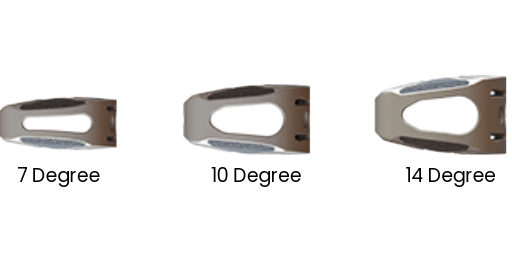
3 LORDOTIC ANGLES
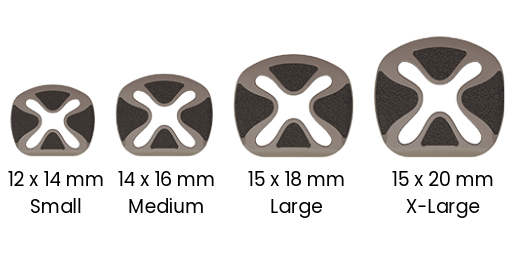
4 FOOTPRINTS
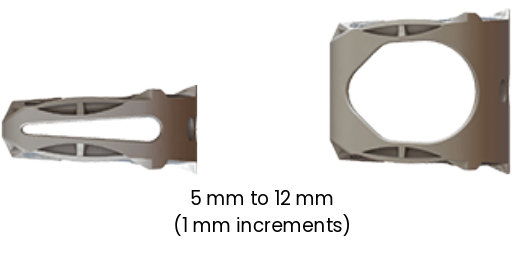
8 HEIGHTS
System Features
Essential features for the next generation of Cervical Interbody Spacers

Patented Lobe design allows for more evenly distributed contact with the vertebral endplates
The convex lobe design of the SureMAX-X™ will match with the concavity of the endplates and will allow for uniform settling of the vertebral bodies.

Convex surfaces to match the spinal endplates
The endplates of the SureMAX™ Family Spacers are convex to match the concavity of the spinal endplates providing greater bone implant interface and stability.

Roughened and optimized acid etched porous surface
Roughened and optimized open-pore structures. The superior and inferior surfaces have a lattice mesh surrounding a large central cavity for placement of bone graft which extends vertically through the implant.

Central cavity for placement of bone graft
The SureMAX™ Family of spacers have a large central graft window as well as side windows to allow for the easy placement of bone graft.

Large lateral windows to facilitate radiographic confirmation of fusion
The lateral aspects of the spacer are open to facilitate radiographic fusion assessment.

Threaded for Attachments
The anterior face incorporates three holes, the center one threaded, for inserter attachment. The inserters have a central threaded rod providing security for greater implant control.

Singular Toolset for SureMAX and SureMAX-X
The SureMAX™ Family of Cervical Spacers utilze a single tray of instruments for simplicity and interoperative options.

SureMAX™ Spacers are provided sterile only
The SureMAX™ Family of spacers are available sterile packed to reduce SPD expenses and provide quick, easy access for unplanned surgeries.
The SureMAX™ Family of Cervical Spacers
The new standard in interbody design.
One set of instruments for both SureMAX™ and SureMAX-X™ Cervical Spacers.
SureMAX-X® cervical spacer instrumentation

Single Modular Tray

Distraction Wedges to help open collapsed disc spaces.

Aggressive Rasps with convex surfaces to strip and shape concavity of vertebral endplates.

Trials match the geometry of the implants for an accurate assessment of size.

Variety of Inserter Tools, with and without guards, allow for single handed tool/implant application.

Patented Slap Hammer design allows rapid insertion/extraction and enhanced visualization of the operative site when using a microscope.
Resources
Brochures & Surgical Technique Guides
Important Information
Description, Indications, and Contraindications
Description
The SureMAX™ Family of Cervical Spacers are interbody fusion devices manufactured from Ti-6Al- 4V ELI titanium alloy (ASTM F3001, Grade 23). The device is designed to provide surgical stabilization of the cervical spine. The superior and inferior surfaces have a lattice mesh surrounding a large central cavity for placement of bone graft which extends vertically through the implant. Three pyramidal spikes extend outward from the implant at the anterior-inferior and anterior-superior edges to aid in securing the device within the disc space. The lateral aspects of the spacer are open to facilitate radiographic fusion assessment. The anterior face incorporates three holes, the center one threaded, for inserter attachment.
The SureMAX™ Cervical Spacer is available in three footprint sizes (depth x width): 12 mm x 14 mm, 14 mm x 16 mm, and 15 mm x 18 mm. All three footprint sizes are available in heights of 5 mm to 12 mm, in 1 mm increments. The superior-inferior surfaces form either a lordotic (7°) or a hyperlordotic (14°) sagittal angulation.
The SureMAX-X™ Cervical Spacer is available in four footprint sizes (depth x width): 12 mm x 14 mm, 14 mm x 16 mm, 15 mm x 18 mm and 15 mm x 20 mm. All four footprint sizes are available in heights of 5 mm to 9 mm, in 1 mm increments. The superior-inferior surfaces form either a lordotic (7°), a mid-lordotic (10°) or a hyperlordotic (14°) sagittal angulation. All the SureMAX™ Family of Cervical Spacers are provided sterile only.
Indications
The SureMAX™ and SureMAX-X™ Cervical Spacers are intended for anterior interbody fusion in skeletally mature patients who have had at least six weeks of non-operative treatment. The SureMAX™ and SureMAX-X™ Cervical Spacers are indicated to treat cervical disc degeneration and/or cervical spinal instability, as confirmed by imaging studies (radiographs, CT, MRI), that results in radiculopathy, myelopathy, and/or pain at multiple contiguous levels from C2 – T1. The SureMAX™ Family of Cervical Spacers are to be used with supplemental fixation; the Hyperlordotic implants (≥ 10°) are required to be used with an anterior cervical plate. The implants are designed for use with autogenous and/or allogeneic bone graft comprised of cancellous and/or cortico-cancellous bone to facilitate fusion.
Contraindications
+ Active local infection in or near the operative region.
+ Active systemic infection and/or disease.
+ Severe osteoporosis or insufficient bone density, which in the medical opinion
of the physician precludes surgery or contraindicates instrumentation.
+ Current metastatic tumors of the vertebrae adjacent to the implant.
+ Known or suspected metal sensitivity.
+ Endocrine or metabolic disorders known to affect osteogenesis
(e.g., Paget’s disease, renal osteodystrophy, hypothyroidism).
+ Significant mental disorder or condition that could compromise the patient’s ability
to remember and comply with preoperative and postoperative instructions.
+ Neuromuscular disorder that would engender unacceptable risk of instability, implant
fixation failure, or complications in postoperative care. Neuromuscular disorders
include spina bifida, cerebral palsy, and multiple sclerosis.
+ Pregnancy.
+ Patients unwilling or unable to follow postoperative instructions,
especially those in athletic and occupational activities.
+ Morbid obesity.
+ Symptomatic cardiac disease.
+ Skeletal immaturity.
+ Grossly distorted anatomy.
+ Any condition not described in the Indications for Use.
Warnings and Precautions
Precautions
+ The implantation of an intervertebral body fusion device should be performed only by experienced spinal surgeons with specific training in the use of this device because this is a technically demanding procedure presenting a risk of serious injury to the patient.
+ Based on the fatigue testing results, the physician/surgeon should consider the level of implantation, patient weight, patient activity level, other patient conditions, etc. which may impact on the performance of the system.
+ The surgeon must have a thorough knowledge of the mechanical and material limitations of surgical implants made of Titanium Alloy and be thoroughly familiar with the surgical technique for implanting the SureMAX™ and SureMAX-X™ Cervical Spacers for the given Indications for Use.
+ The surgeon should be familiar with the various devices and instruments and verify that all are available before beginning the surgery. Additionally, the packaging and implant should be inspected for damage prior to implantation.
+ In the event that removal of the implant is considered (e.g. due to loosening, fracture, migration of the implant; infection; increased pain, etc.), the risks versus benefits must be carefully weighed. Such events can occur even after healing, especially in more active
patients. Appropriate postoperative care must be given following implant removal to avoid further complication.
+ The surgeon must be thoroughly familiar with the options for supplemental internal fixation systems and the associated surgical techniques.
+ Implants must be fully seated within the inserter prior to use. Care must be taken not to over-tighten the implant-inserter assembly. Additionally, care must be taken not to manipulate the inserter implant interface in a way not recommended by the surgical technique.
+ The surgeon must ensure that the implant is properly seated prior to closing of the soft tissue.
+ Extreme caution must be used around the spinal cord, nerve roots and blood vessels.
MRI Safety Information
The SureMAX™ Family of Cervical Spacers have not been evaluated for safety and compatibility in the MR environment. It has not been tested for heating, migration, or image artifact in the MR environment. The safety of these Cervical Spacers in the MR environment is unknown. Scanning a patient who has this device may result in patient injury.
Potential Adverse Effects
Potential complications and adverse effects for this system are similar to those of other spinal instrumentation systems and include, but are not limited to: pseudarthrosis, insufficient bone stock, painful bursa, pressure necrosis, palpable components, early or late loosening of the components; disassembly, bending or breakage of any or all of the components; foreign body (allergic) reaction to the implants; infections possible requiring removal of devices; loss of neurological function, including paralysis, spinal cord impingement or damage.


